Maxwell’s energy distribution law:
Let us consider a system of an ideal Boltzmann gas of \(N\) molecules of mass \(m\) in a volume \(V\).
Let us consider a continuous distribution of the molecular energies so that the energy levels are continuously distributed. The number of the molecules between the energy range \(\epsilon\) and \(\epsilon+d\epsilon\) is given by,
\(\displaystyle{n(\epsilon)\ d\epsilon=f(\epsilon)\ g(\epsilon)\ d\epsilon=\frac{g(\epsilon)\ d\epsilon}{e^{\alpha+\frac{\epsilon}{kT}}}}\tag{1}\)where, \(\displaystyle{f(\epsilon)=\frac{1}{e^{\alpha+\frac{\epsilon}{kT}}}}\) is the Maxwell-Boltzmann Distribution function.
Now, the phase sphere volume in the momentum range \(p\) and \(p+dp\) is given by,
\(\displaystyle{\iiint}{dq_x}\cdot{dq_y}\cdot{dq_z}\ \displaystyle{\iiint_{p}^{p+dp}}{dp_x}\cdot{dp_y}\cdot{dp_z}\\=V\cdot{4\pi}p^2\ dp\)where, \(\displaystyle{\iiint}{dq_x}\cdot{dq_y}\cdot{dq_z}=V\), the co-ordinate volume.
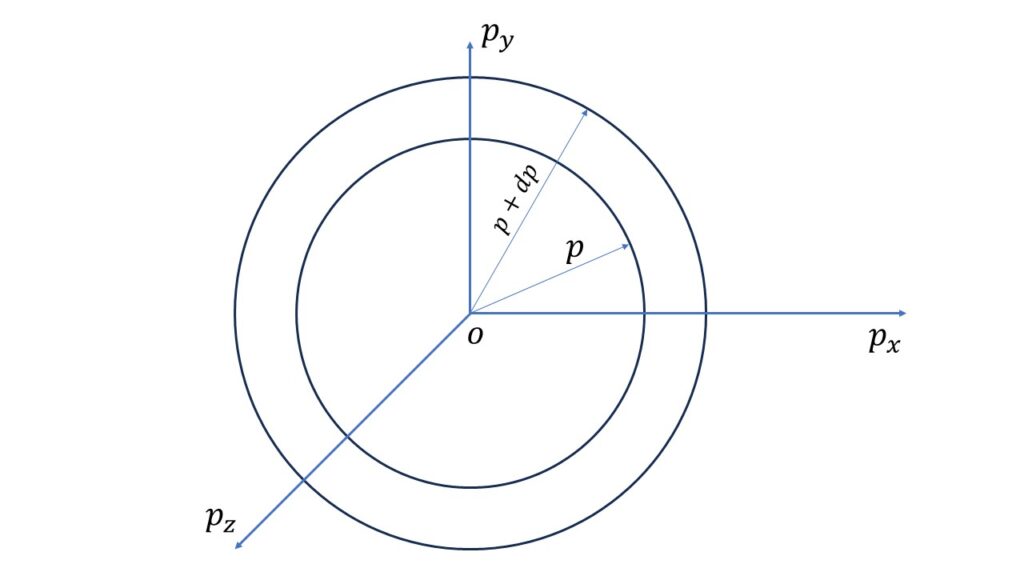
So the number of the quantum states in the energy range \(\epsilon\) and \(\epsilon+d\epsilon\) is given by,
\(\displaystyle{g(\epsilon)\ d\epsilon=\frac{V\cdot{4\pi}p^2{dp}}{h^3}}\),
where \(h^3=dq_x\ dq_y\ dq_z\ \cdot\ dp_x\ dp_y\ dp_z\) is the volume of each quantum state.
\(\displaystyle{=\frac{4\pi{V}(\sqrt{2m\epsilon})^2\cdot\ d\sqrt{2m\epsilon}}{h^3}}\), where \(p=\sqrt{2m\epsilon}\)
\(\displaystyle{=\frac{4\pi{V}\cdot{2m\epsilon}\sqrt{2m}{\epsilon}^{-1/2}d\epsilon}{h^3}}\)
\(\displaystyle{=\frac{2\pi{V}{(2m)}^{3/2}{\epsilon}^{1/2}d\epsilon}{h^3}}\tag{2}\)Using (2) in (1) we get,
\(\displaystyle{n(\epsilon)\ d\epsilon=\frac{2\pi{V}{(2m)}^{3/2}{\epsilon}^{1/2}e^{-\alpha}e^{-\epsilon/kT}d\epsilon}{h^3}}\tag{3}\)In order to find the value of \(e^{-\alpha}\), we use the relation
\(N=\displaystyle{\int_{0}^{\infty}}n(\epsilon)\ d\epsilon\) \(\displaystyle{=\frac{4\pi{V}\cdot{2m}^{3/2}}{h^3}e^{-\alpha}\int_{0}^{\infty}{\epsilon}^{1/2}\cdot{e^{-\epsilon/kT}}\ d\epsilon}\) \(\displaystyle{=\frac{4\pi{V}\cdot{2m}^{3/2}}{h^3}e^{-\alpha}{(kT)}^{1/2}\cdot{kT}\cdot\int{x}^{1/2}\cdot{e}^{-x}\ dx}\)[putting \(\frac{\epsilon}{kT}=x,\ or,\ d\epsilon=kT\ dx\)]
\(\displaystyle{=\frac{4\pi{V}\cdot{2m}^{3/2}}{h^3}e^{-\alpha}{(kT)}^{3/2}\ \Gamma{(3/2)}}\) \(\displaystyle{=\frac{4\pi{V}\cdot{2m}^{3/2}}{h^3}e^{-\alpha}{(kT)}^{3/2}\frac{1}{2}\sqrt{\pi}}\) \(\displaystyle{=\frac{V\cdot{(2m\pi{kT})}^{3/2}\cdot{e^{-\alpha}}}{h^3}}\)Therefore, \(\displaystyle{e^{-\alpha}=\frac{Nh^3}{V}\frac{1}{{(2m\pi{kT})}^{3/2}}}\)
Now putting the value of \(e^{-\alpha}\) in equation (3) we get,
\(\displaystyle{n(\epsilon)\ d\epsilon=\frac{4\pi{V}\cdot{2m}^{3/2}}{h^3}\cdot{\frac{Nh^3}{V}\frac{1}{{(2m\pi{kT})}^{3/2}}}{\epsilon}^{1/2}e^{-\epsilon/kT}\ d\epsilon}\)
therefore,
\(\displaystyle{n(\epsilon)\ d\epsilon=2\pi{N}{\left(\frac{1}{\pi{kT}}\right)}^{3/2}{\epsilon}^{1/2}e^{-\epsilon/kT}\ d\epsilon}\tag{4}\)Maxwell’s velocity distribution law:
Putting \(\displaystyle{\epsilon=\frac{1}{2}mc^2}\) and \(\displaystyle{d\epsilon=m\ c\ dc}\) where c is the resultant speed of a molecule.
We get from equation (4), the number of the particles having velocities in the range \(c\) and \(c+dc\) is
\(\displaystyle{n(c)\ dc=2\pi{N}{\left(\frac{1}{\pi{kT}}\right)}^{3/2}{\left(\frac{1}{2}mc^2\right)}^{1/2}e^{-\frac{mc^2}{2kT}}mc\ dc}\) \(or,\ \displaystyle{n(c)\ dc=4\pi{N}{\left(\frac{m}{2\pi{kT}}\right)}^{3/2}\cdot{c^2}e^{-\frac{mc^2}{2kT}}\cdot\ dc}\tag{5}\)This is the Maxwell’s velocity distribution law.
Maxwell’s momentum distribution law:
Putting \(\displaystyle{\epsilon=\frac{p^2}{2m}}\) and \(\displaystyle{d\epsilon=\frac{p}{m}dp}\) in equation (4) we get the number of particles having momenta between \(p\) and \(p+dp\) is
\(\displaystyle{n(p)dp=4\pi{N}{\left(\frac{1}{\pi{kT}}\right)}^{3/2}{\left(\frac{p^2}{2m}\right)}^{1/2}e^{-\frac{p^2}{2mkT}}\frac{p}{m}\ dp}\)
therefore,
\(\displaystyle{n(p)dp=4\pi{N}{\left(\frac{1}{2\pi{mkT}}\right)}^{3/2}p^2e^{-\frac{p^2}{2mkT}}\ dp}\tag{6}\)
This is Maxwell’s momentum distribution law.